Steris provides a variety of products and services to the healthcare industry including to hospitals, medical device manufacturers, pharmaceutical companies and biotechnology businesses, as well as food safety and industrial markets. The company’s main areas of activity are providing hygiene, sterilisation and anti-microbial treatment services to these end markets in order to ensure a safe and hygienic operating environment.
Objective
Strengthen our understanding of the positive impact associated with Steris’ products and services.
Background/issue
We have been speaking to investee companies as part of a project to further strengthen our understanding of the positive impact associated with their products and services. Additional insights allow us to build a stronger ‘impact investment case’ for each of our stocks helping us to deepen our analysis and refine the impact score we give to our companies.
Actions
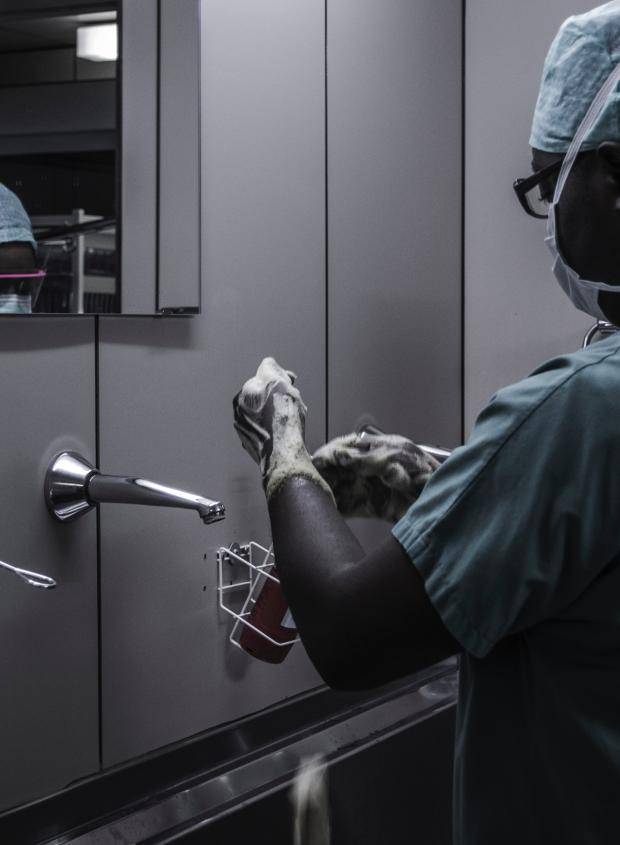
Whilst undertaking a review of the research within Steris’ Impact Engine, we identified areas that would benefit from additional detail, including quantifying the extent to which human error influences the efficacy of sterilisation and reprocessing of medical devices, the role of Steris’s products and services in enabling the positive outcomes, and the uniqueness of the company’s contribution.
Outcomes
Successful/Milestone 2: We had a productive call with our contact in Investor Relations who provided additional detail for our impact analysis. For example, we discussed the role of human error as the most significant factor influencing the efficacy of Steris’s equipment and the measures taken by the company to reduce the risk of it occurring. This includes reducing the number of decision and touch points for reprocessing staff by increasing automation. The company was, however, unable to quantify the proportion of processes that have been automated. We also discussed Steris’ in-house training provided for the reprocessing operatives working within its outsourcing team and the technologies that it uses to ensure that processes run correctly.
In terms of other factors or services influencing the efficacy of the sterilisation process, Steris was keen to point out that hospital-acquired infections do not typically come from equipment but, instead, mostly from poor hand hygiene or improper cleaning of the operating room though the company did not have any data on this. Steris had previously made efforts to offer sterilisation for touchpoint items like blood pressure cuffs, for example, through the use of hydrogen peroxide chambers, though, this was not taken up by the industry because it slows the turnover of the equipment.
This information has been helpful for adding additional detail in understanding the positive impact of Steris’s products, however it did not change any of the underlying scores within our impact analysis.