Objective
For Gerresheimer to publish data that helps to strengthen investors’ understanding of the positive impacts enabled through its products and services.
Background
We continually seek to further strengthen our understanding of the positive and negative impacts associated with the products and services sold by our investee companies. Additional insights allow us to build stronger investment conviction in a stock and more clearly set out the ‘impact story’. Equally, understanding negative impacts is an essential step towards addressing these impacts.
Actions
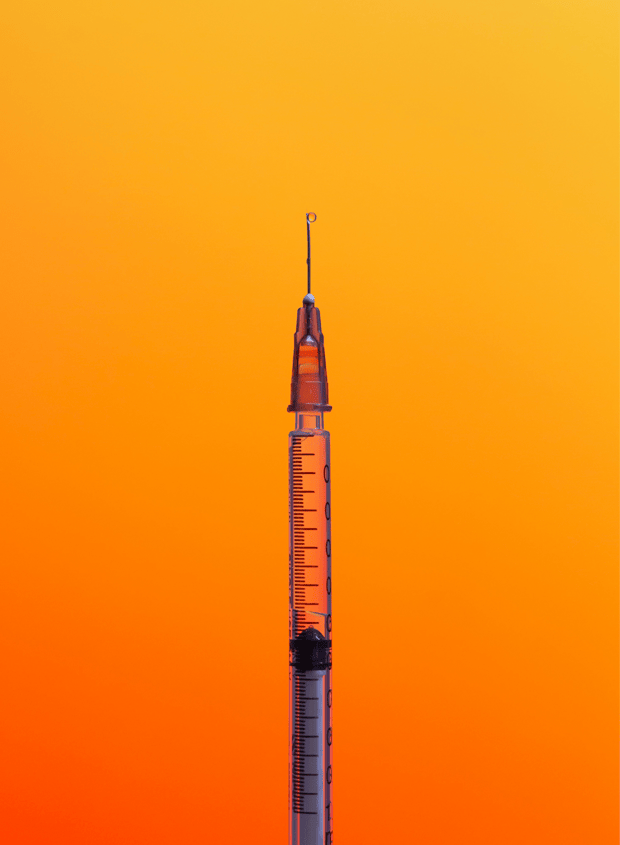
During a call with Gerresheimer we raised the issue of product impact. Specifically, we focused on the environmental impact of their auto-injectors and pens, particularly around end-of-life disposal.
Outcomes
M2
Gerresheimer confirmed that this issue is firmly on their agenda, and they are actively in discussions with customers to address the challenges. However, several factors make this a complex problem:
Regulatory Challenges: The pharmaceutical industry is highly regulated, and any changes to how used auto-injectors are handled must comply with strict standards. This makes it difficult to implement widespread changes quickly.
Hazardous Waste Management: Used auto-injectors are considered hazardous waste, meaning that they must be dismantled and disposed of in separate parts, which complicates the process of managing these devices responsibly.
Logistical Barriers: There are significant logistical challenges in setting up a system for returning used injectors. GXI is evaluating options for collection points, such as pharmacies or doctor's offices, but these considerations require further analysis and planning.
Gerresheimer also acknowledged that multiple-use injectors could reduce waste, although they don't completely eliminate the issue. As the market for auto-injectors is growing, the company recognises that this will become a more pressing topic in the near future and is committed to finding solutions. They are actively exploring product lifecycle improvements, but significant barriers remain that will require innovative approaches and collaboration within the industry. We will continue to raise this topic with Gerresheimer during our meetings with the company, but ultimately advancements will need to be made in collaboration with pharmaceutical companies and the FDA.
Gerresheimer
Q2 2024 Engagement case study: Product impact (end of life)