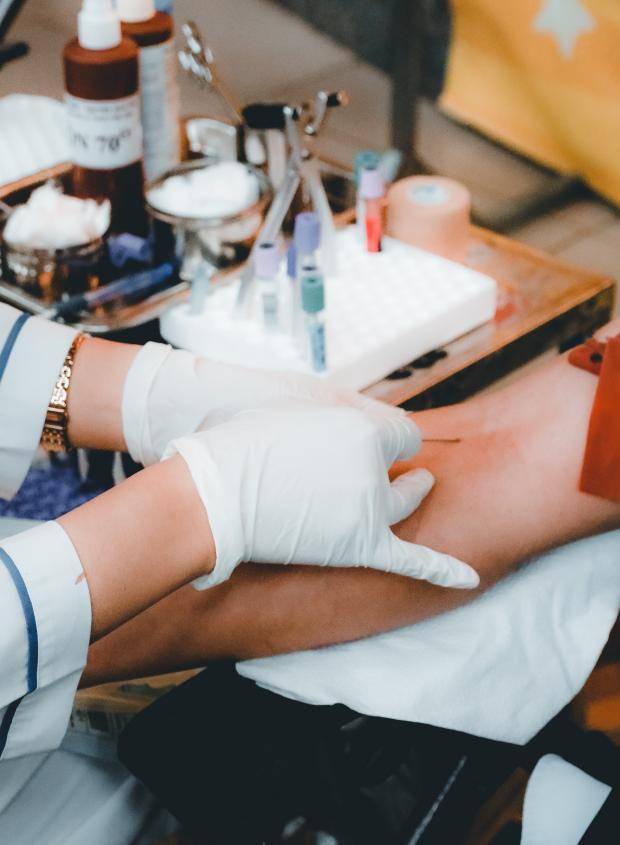
CSL is a provider of human blood-plasma derived products to treat bleeding disorders, rare and serious infections and autoimmune diseases. CSL also manufactures vaccines and related products, including for flu and cervical cancer, as well as other products that speed up recovery times for patients that have undergone heart surgery, organ transplants and burns.
Objective:
- Understand ethical implications around frequency of blood plasma collections.
- To improve the donor experience, especially in terms of non-financial value add.
Background:
WHEB has been engaged in ongoing dialogue with CSL on the ethical implications of blood plasma donation, known as plasmapheresis, since 2017.
As investors, we regard CSL very highly. We appreciate that blood plasma products are lifesaving, while the vaccines developed by the company play a critical role in preventing illness. We are also mindful that paid for donations are essential for ensuring global supplies, but often questioned in the media, making the conversation both complex and nuanced.
Since the COVID-19 pandemic we have also been aware of increased competition for donors, placing pressure on margins and making improved donor experience more important than ever.
Actions:
In Q3 2022, WHEB was contacted by an Australian investor with questions concerning the frequency with which CSL enables people to give blood plasma, known as plasmapheresis, and about the ethical implications of doing so.
Given our history engaging CSL on this topic, we were able to share some initial context. For example, CSL’s franchise critically relies on the long-term safety of donors. Also, plasmapheresis, in which only proteins are lost, enables more frequent donation than when giving whole blood, which requires replacement of blood and plasma.
Considering the increased complexity of the issue and pressure on margins in light of the COVID-19 pandemic, we undertook further research both independently and by speaking to CSL. Initially we exchanged emails with CSL’s Investor Relations team in which donor screening protocols, benefits (financial and non-financial) and donation frequency were discussed.
These exchanges reaffirmed our confidence in CSL’s focus on product, and therefore donor, safety, though we also emphasised our desire for evidence of consideration of non-financial value-add for donors. We suggested that this could be, for example, through comprehensive wellbeing assessments that are easily shared with healthcare providers.
Outcome/Investor Contribution:
M3 - Company develops or commits to developing an appropriate policy or strategy to manage the issue
Dialogue developed into a lengthy call with the company’s CSL’s investor relations team and an internal plasma expert in Q3 2022. Ultimately, since the pandemic, a significant (c.20%) increase in donor competition has inflated the market donation rate leading CSL to implement other measures such as:
- Adoption of the RIKA plasma donation system, which provides improved comfort in the donation process.
- Investment into an App aimed at reductions in donation time reducing potential adverse effects to donation.
CSL’s has also undertaken research with the Plasma Protein Therapeutics Association which has helped conclude US donation frequency does not adversely impact health.
Although not a treatment organization, CSL’s business model inherently looks to reduce referral rates. On this basis, it is looking at how to support donor treatment access, which then enables donation, without becoming a treatment organization itself.